
Potassium Tartrate

Specifications
| Item | Index |
| Purity ≥% | 99.5 |
| Chlorie(Cl)≤% | 0.01 |
| Heavy metal(Pb)≤% | 0.002 |
| Sulfate(SO4)≤% | 0.015 |
| Iron(Fe)≤% | 0.005 |
| Phosphate(PO4)≤% | 0.005 |
| Transparency | qualified |
Packing & Storage
| Packing | in 25kg bag |
| Storage | 20℃, 2 years. |
| Shipping | Room temperature in China; may vary elsewhere |
Free Quote
For samples, pricing, or more information, please call us at 0086-25-52397805 or mail to info@liwei-chem.com or fill out the following form. We will respond to you as soon as possible.
Tel: 0086-25-52397805
E-mail: info@liwei-chem.com
E-mail: sophiahoney247@gmail.com


General Information
| Common Names | Potassium Tartrate | POTASSIUM L-BITARTRATE | ||||||
| Structure | 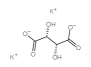 |
||||||
| CAS No. | 921-53-9,304655-91-2 | Boiling Point (℃) | 399.3ºC | ||||
| Molecular Weight | 226.268 | Melting Point (℃) | N/A | ||||
| Appearance | White powder | Vapor Specific Gravity | N/A | ||||
| HS Code | 2918130000 | Flash Point (℃) | 209.4ºC | ||||
| Solubility | The solubility in water varies with temperature, insoluble in ethanol, acetic acid, easily soluble in inorganic acid | Autoignition Temperature (℃) | |||||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
1. According to the characteristics that potassium hydrogen tartrate is soluble in hot water, first add soft water or distilled water twice as much as tartar or acid-reducing precipitate to the reactor, heat to boiling, start stirring and add tartar or acid-reducing precipitate Precipitate. After all the materials are dissolved, add solid potassium carbonate. Since the reaction is more intense, and a large amount of carbon dioxide gas is produced during the reaction, in order to make the operation stable, when adding potassium carbonate, it should be added in batches and the stirring should be strengthened. The addition of salt of wormwood stops when the pH value of the system is 7 with litmus paper test.
2. Filter the neutral reaction material while it is hot, heat the filtrate to boiling, and add activated carbon for decolorization. Activated carbon and other impurities are then removed by filtration. The amount of activated carbon added is based on the colorless and transparent material, generally 5%~10% of the material.
3. Heat and evaporate the decolorized material. When the concentration of potassium tartrate reaches the requirement, put the material into an enamel plate, and cool and crystallize naturally at room temperature.
4. Dry the crystals to obtain potassium tartrate with a content of more than 99%.
Frequently Asked Questions
Application of Potassium Tartrate
Potassium tartrate is widely used in food, medicine, chemical industry, light industry and other industries, mainly for the manufacture of tartrates, such as antimony potassium tartrate, potassium sodium tartrate, etc. Potassium tartrate is used in the food industry as beer foaming agent, food sour agent, flavoring agent, etc. The sour taste of potassium tartrate is 1.3 times that of citric acid, and it is especially suitable as a sour agent for grape juice. This product is identified as an excellent food additive by the FAO/WHO expert committee. Potassium tartrate also plays a very important role in industries such as leather tanning, photography, glass, enamel, and telecommunications equipment.
