
D(+)-Tartaric Acid

Specifications
| Item | Index |
| Content | 99.5% Min |
| Specific Rotation [α]D25℃ | -12°~-12.8° |
| Drying loss | 0.3% Max |
| Sulfate | 0.05% Max |
| Heavy Metal(as Pb) | 0.001% Max |
| Ignition residue | 0.1% Max |
| Optical purity | 99.7% Max |
Packing & Storage
| Packing | in 25kg bag |
| Storage | 20℃, 2 years. |
| Shipping | Room temperature in China; may vary elsewhere |
Free Quote
For samples, pricing, or more information, please call us at 0086-25-52397805 or mail to info@liwei-chem.com or fill out the following form. We will respond to you as soon as possible.
Tel: 0086-25-52397805
E-mail: info@liwei-chem.com
E-mail: sophiahoney247@gmail.com


General Information
| Common Names | D(+)-Tartaric Acid | Butanedioic acid, 2,3-dihydroxy-, (2S,3S)- | (-)-tartaric acid | ||||||
| Structure | 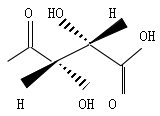 | ||||||
| CAS No. | 147-71-7 | Boiling Point (℃) | 399.3±42.0 °C | ||||
| Molecular Weight | 150.087 | Melting Point (℃) | 166-170ºC | ||||
| Appearance | White powder | Vapor Specific Gravity | 1.9±0.1 g/cm3 | ||||
| HS Code | 2918120000 | Flash Point (℃) | 209.4±24.4 °C | ||||
| Solubility | Soluble in water, methanol, propyl alcohol, glycerol and ethanol, insoluble in chloroform | Autoignition Temperature (℃) | |||||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Skin | Redness. Burning sensation. Itching. | Protective gloves. | Remove contaminated clothes. Rinse and then wash skin with water and soap. |
| Eyes | Redness. Pain. | Wear safety goggles. | First rinse with plenty of water for several minutes (remove contact lenses if easily possible), then refer for medical attention. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
Frequently Asked Questions
Chemical Properties
Tartaric acid exists in three stereoisomers: tartaric acid, l-tartaric acid, and meso-tartaric acid. The optical activity of the mixture of equal amounts of D-isomer and L-isomer cancels each other out, which is called racemic tartaric acid. The meso body does not exist in nature and can be synthesized chemically. All tartaric acids are colourless crystals that are readily soluble in water.
Use
Tartaric acid is widely used as an acidulant in beverages and other foods, similar to citric acid. Tartaric acid is used in combination with tannins as a mordant for acid dyes and is also used in some developing and fixing operations in the photographic industry. Its iron salts are photosensitizing, so they can be used to make blueprints. Tartaric acid can be complex with various metal ions and can be used as a cleaning and polishing agent for metal surfaces. Potassium sodium tartrate (Rochelle’s salt) can be used to prepare Fehling’s reagent. It can also be used in medicine as a laxative, diuretic, and intermediate of Cincofen. Its crystal has piezoelectric properties and can be used in the electronics industry.
It is mainly used as a sour agent, biochemical reagent, racemate resolving agent and pharmaceutical resolving agent in beverages, candy, bread and gelatin. It is also used in photography, canning, printing and dyeing, pottery, and preparation of other resolving agents, salts, esters, etc. and as a pharmaceutical resolving agent, food additive, biochemical reagent, etc.
